UK ISRCTN 98886991
UK IRAS306761
UK CPMS50913
WHO UTN U1111-1273-9942
https://stroke.nottingham.ac.uk/pheast/live/
Blank EOI Form (PDF)We would love to have you on board!
| Title | Pharyngeal Electrical Stimulation for Acute Stroke dysphagia Trial |
| Acronym | PhEAST |
| Short title | Pharyngeal Electrical Stimulation for Post Stroke dysphagia |
| Chief investigator | Professor Philip M Bath |
| Objectives |
Overall
|
| Trial configuration | International prospective randomised open-label blinded-endpoint (PROBE) parallel group superiority phase IV effectiveness trial |
| Setting | Secondary care: acute stroke services |
| Sample size estimate |
The null hypothesis is that PES does not alter DSRS at day 14 post recruitment in participants with PSD. The primary outcome, DSRS (dysphagia severity rating scale) will be compared between PES and no PES using multiple linear regression with adjustment for stratification and minimisation variables. Assuming alpha 5%, power 90%; DSRS difference 1.2, standard deviation 5.0; losses 3%, crossovers 3%; sample rounded up, a sample size of N=800 is needed (PES n=400, control n=400) (assumptions based on pilot trials and STEPS trial). |
| Number of participants | 800 |
| Eligibility criteria |
Inclusion:
800 hospitalised adults (age ≥18) with recent (2-31 days) ischaemic or haemorrhagic anterior or posterior circulation stroke (as diagnosed clinico-radiologically) at a stroke centre, and Clinical dysphagia defined as a functional oral intake scale (FOIS) score of 1 (nothing by mouth, feeding by NGT/PEG),2 (tube dependent with minimal attempts of food or liquids) or 3 (tube dependent with consistent oral intake of food or liquids). NIHSS item 1a score score of 1 (nothing by mouth, feeding by NGT/PEG),2 (tube dependent of 0, 1 or 2 (where the patient requires repeated stimulation to arouse). Baseline DSRS supervision score of either 3 (requiring therapeutic feeding by SALT team; on oral trials) or 4 (No oral feeding). Excluded: Non-stroke dysphagia, e.g., due to traumatic brain haemorrhage, subarachnoid haemorrhage, brain tumour, Parkinson’s disease, multiple sclerosis, severe dementia, head or neck cancer. NIHSS item 1a score of 2 (where the patient only responds to pain) or NIHSS item 1a score of 3. Pre-stroke dysphagia or dependency (modified Rankin scale, mRS 4/5). Investigator believes dysphagia will be short-term, e.g. there are signs of impending recovery in swallowing (i.e. enrolment is reserved for patients whom the investigator considers are unlikely to resolve spontaneously over the next few days). Patient expected to be repatriated to a separate organisation. Patient expected to be rehabilitated at a separate organisation. Patient not likely to be in the treating hospital for at least 14 days. Ongoing or anticipated ventilation/intubation/tracheostomy. Ongoing treatment of dysphagia with other forms of electrical / magnetic stimulation (e.g. NMES, TCDS, rTMS or Ampcare), or devices (e.g. EMST, IQORO, IOPI, biofeedback that uses EMG electrodes, or chin tuck against resistance using a ball/chin depressor) – this applies for the duration of the trial Malignant middle cerebral artery syndrome. Pregnant. Pacemaker, cochlear implant or implantable cardioverter-defibrillator. Need for >35% of oxygen. Two or more NGT pulled out within the last week unless nasal bridle in place. Investigator feels patient will not tolerate PES catheter. Palliative care. Presence of a pharyngeal pouch. Participant is risk-feeding at time of screening. |
| Description of interventions |
Intervention arm
PES on top of guideline-based standard-of-care. PES will be administered on days 1-6 using a commercial catheter with integral feeding tube. PES involves six daily 10 minute treatments at 5 Hz; threshold and tolerability currents will be assessed and the treatment current set at threshold + 0.75 x (tolerability - threshold) with current generated by a base-station. Dosing levels will be monitored, and sites informed if the stimulation current is too low, i.e. <20 mA; sites will be re-trained on the importance of delivering adequate current, if necessary. The catheter will be replaced if removed or pulled out prior to 6 treatments being administered. Treatment will be administered by PES-trained research coordinators, nurses or SLTs who are not involved in outcome data collection. Comparator armNo PES catheter/stimulation on top of best guideline-based standard-of-care dysphagia management. A standard NGT will be used for feeding as necessary. |
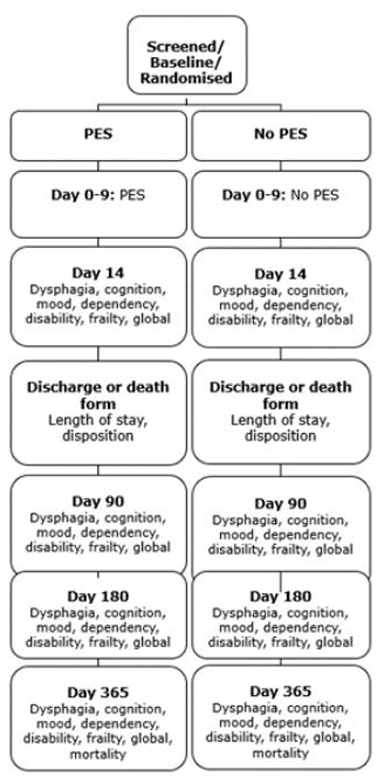
| Duration of study | From randomisation: treatment for 6 days, primary outcome at day 14, follow-ups done by coordinating centre at day 90, day 180 and day 365. |
| Randomisation and blinding | Randomised 1:1 with minimisation on age, sex, dysphagia severity rating scale (DSRS), impairment (National Institutes of Health stroke scale [NIHSS]), stroke type (ischaemic/haemorrhagic), circulation (anterior/posterior), time to randomisation, country (UK vs non-UK); with 5% simple randomisation. Treatment will be delivered by a dedicated member of staff not otherwise involved in the blinded outcomes. Outcomes will be assessed by other staff blinded to randomised treatment. |
| Outcome measures |
Primary at day 14−1 +3 (day 13-17)
Secondary at days 1-9
Secondary at day 14−1 +3 (day 13-17) Note 1: We have included several measures of dysphagia and feeding: DSRS, FOIS, EAT-10 and FSS to triangulate the presence and magnitude of dysphagia and effects on feeding. These will be analysed together using the Wei-Lachin test.
Secondary at discharge/death by hospital assessor:
Secondary at day 90±7 by central telephone assessor blinded to
baseline, treatment and in-hospital data (or by post)*:
Secondary at day 180±7 by central telephone assessor blinded to
baseline, treatment and in-hospital data (or by post)*:
Secondary at day 365±7 by central telephone assessor blinded
to baseline, treatment and in-hospital data (or by post)*:
Safety
Costs |
| Statistical methods |
The primary outcome, DSRS, will be compared between PES and no PES using multiple linear regression with adjustment for minimisation variables. The null hypothesis is that PES does not alter DSRS at day 14 in participants with PSD. Assuming alpha 5%, power 90%; DSRS difference 1.2, standard deviation 5.0; losses 3%, crossovers 3%; sample rounded up, a sample size of N=800 is needed (PES n=400, control n=400). |
PhEAST investigator materials
| Trial |
Description |
| MAPS-2 | Metoclopramide for Avoiding Pneumonia after Stroke 2 |
| ReCAST-3 | Remote ischaemic Conditioning After Stroke Trial 3 |
| TICH-3 | Tranexamic acid for IntraCerebral Haemorrhage 3 |
| GEKO | GEKOTM Venous Thromboembolism Prevention Study |
Philip Bath is Stroke Association Professor of Stroke Medicine, as well as Chair and Head of the Division of Clinical Neuroscience at the University of Nottingham, and Chief Investigator of PhEAST. He has extensive experience in a wide range of large national and international stroke studies and is leading international collaborations on improving the design and analysis of stroke studies.

I am a Senior Clinical Trial Manager within the Stroke Trials Unit, University of Nottingham, based at Queen's Medical Centre. I have 22 years' experience in research, as both a scientist and in research management. My background is somewhat varied, having worked in Pharma, Clinical Trials as well as many years in University research and teaching, in the fields of oncology, respiratory, diabetes and virology medical research, as well as a background in biochemistry, molecular and microbiology. I am working on the trials: MAPS-2 and PhEAST.

I graduated with a Master's degree in 2017 and joined the Oncology Research team at Nottingham University Hospitals NHS Trust shortly after. There I was responsible for facilitating the setup of new, exciting clinical trials for cancers of all types. I left the NHS in December 2020 and have been employed by the University of Nottingham since the beginning of 2021. I hope I can bring all the skills I have learnt to stroke clinical research.

I joined the PhEAST team as International Trial Manager in July 2024. Prior to this I had been working within stroke delivery team at Nottingham since 2012. I have experience of delivering PES treatment and recruiting into PhEAST as well as experience working on older PES trials including STEPS and PHADER

I joined the PhEAST team back in 2023. I am the Research Coordinator for both the PhEAST and TICH-3 (International) trials.


I am a web application/database programmer for PhEAST. My role is to develop and support software and systems for clinical trials, primarily relating to stroke research.
For any queries, please email us.