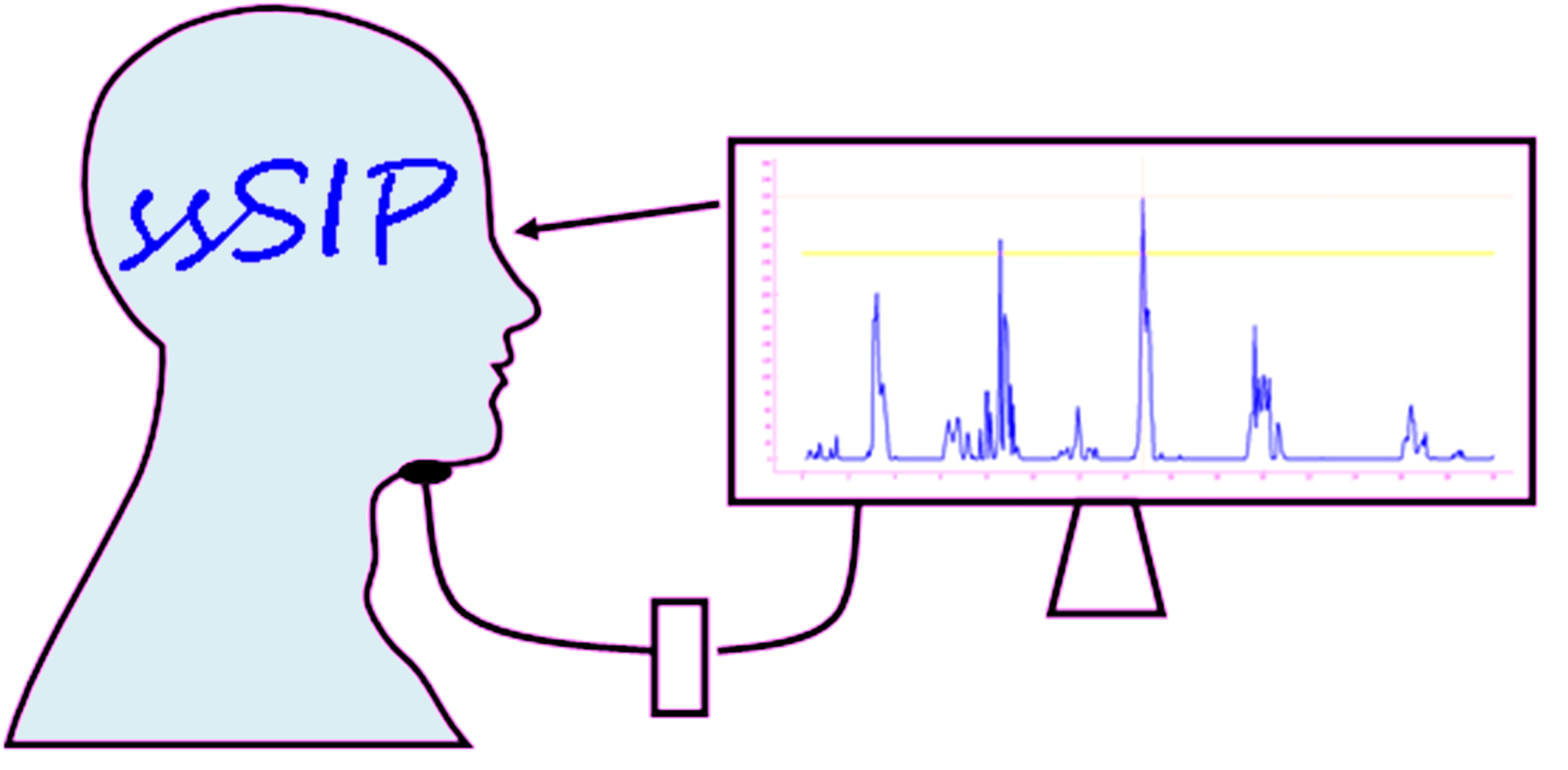
ssSIP trialSwallow Strength and Skill training with biofeedback In Post-stroke dysphagia |
|
Trial documents |
|
ssSIP trialSwallow Strength and Skill training with biofeedback In Post-stroke dysphagia |
|
Trial documents |

|